1 4.3 How Do Cloud Droplets Occur?
How Do Cloud Droplets Occur?
What is the difference between water vapor and a cloud droplet? The difference is one is a gas and the other is a liquid. For a liquid cloud droplet to form, the air must first reach saturation and then condensation can occur. Unfortunately, condensation does not happen spontaneously. Specific conditions must be present for condensation to occur. This submodule examines processes that lead to saturation. Once saturation is present then we will examine the conditions leading to condensation.
4.3.1 Saturation
Three processes in the atmosphere can cause saturation:
- Cooling the air temperature to the dew point. This process is the most common.
- Mixing cold air with warm, moist air.
- Adding moisture (water vapor) to the air. Evaporation is the primary mechanism for this process, which is linked to precipitation.
Lowering air temperature to the dew point
An example of lowering the air temperature to the dew point is air rising over a mountain. As the air flows over the mountain, its altitude increases, and the temperature decreases. In this picture, saturation has occurred along with condensation to form a cloud as the air moves over the mountain.

Mixing cold air with warm, moist air
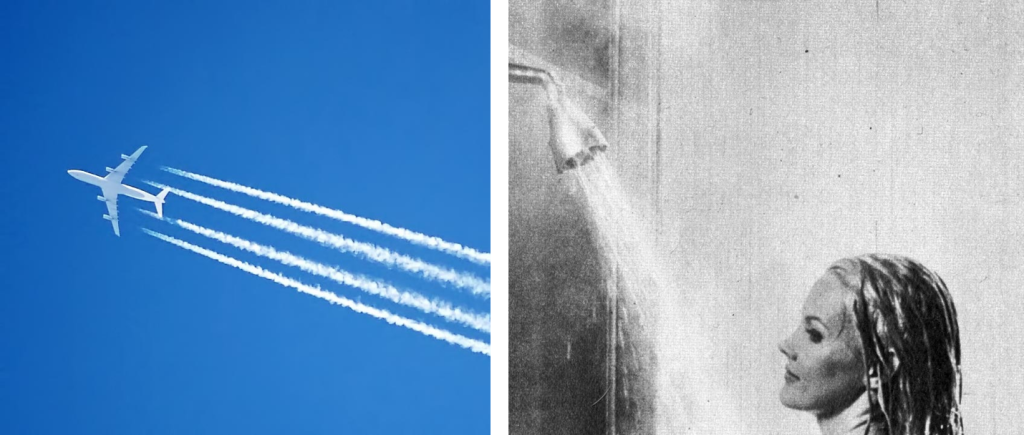
A classic example of mixing cold air with warm, moist air is present in the condensation trail seen behind a jet plane. Combustion of fuel in a jet engine creates exhaust and warm, moist air. This water vapor is much warmer than the surrounding environment, so saturation occurs rapidly followed by condensation.
Adding water vapor to the air
An example of adding water vapor to the air is when a person takes a shower. The shower puts water vapor into the air. Saturation in the room can be reached and condensation starts to occur on the mirror and walls. Rain is the most common method for adding moisture to the air. Obviously, water vapor needs to be in the air before rain can occur, and this entire process of evaporation and precipitation is called the hydrologic (water) cycle.
4.3.2 Factors Affecting Condensation (Liquid Water)
After the atmosphere is saturated (the relative humidity equals 100%), two additional factors affect whether condensation occurs. These factors are (1) supersaturation conditions and (2) the presence of cloud condensation nuclei.
As a water droplet begins to form, both the evaporation and condensation rates influence it. The water molecules on the surface of the droplet are affected by these rates. The molecules inside the droplet are somewhat isolated from these conditions.
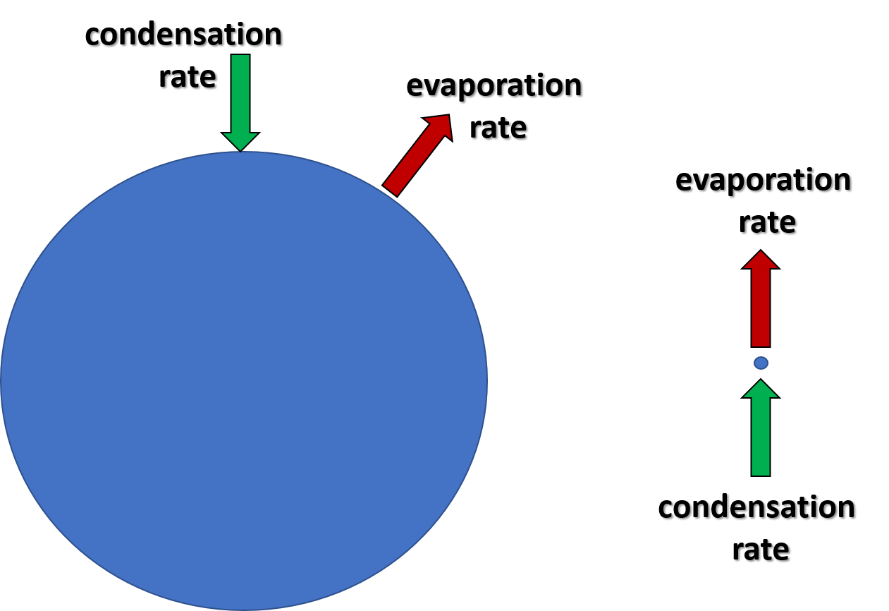
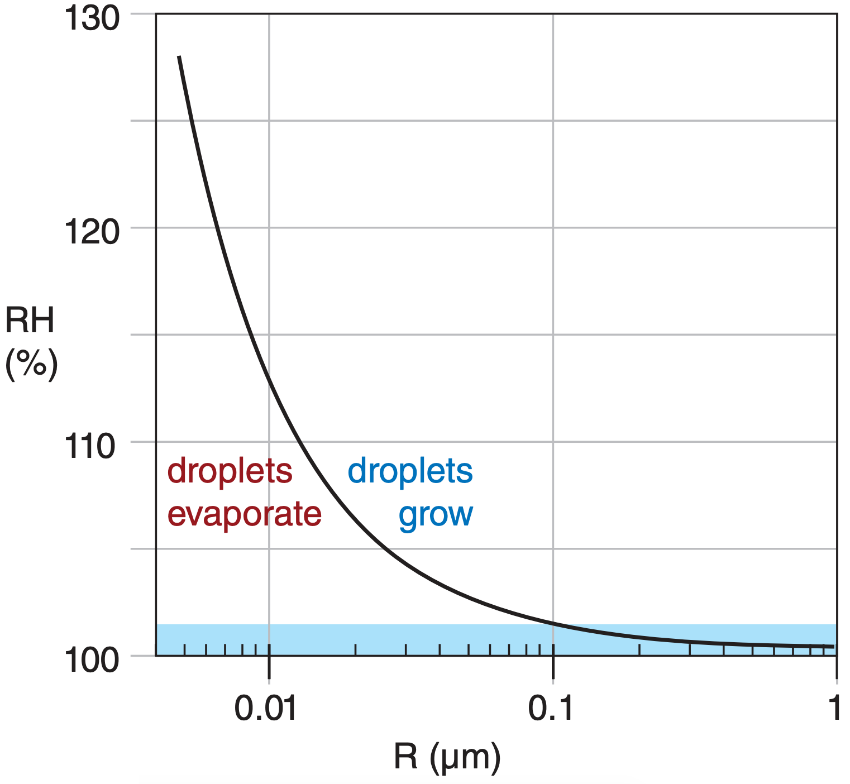
Thus, smaller droplets can easily evaporate and disappear. Larger droplets are much more likely to remain droplets. For a droplet to reach a sustainable size, supersaturated conditions must be present for smaller droplets to grow larger. Supersaturation is defined as relative humidity greater than 100%. In other words, the evaporation rate must be reduced and the condensation rate increased. Relative humidity higher than 100% provides this environment.
This graph shows that droplets less than 1 µm in size require a supersaturation environment to grow. If supersaturation is not present the droplet evaporates. The blue shaded region highlights the range of humidity normally found in clouds.
Water vapor molecules by themselves tend not to condense into a liquid when cooled. The keywords in this sentence are “by themselves”. If a chamber is filled with pure water vapor and nothing else, relative humidity of 300% or higher is present before condensation starts. The atmosphere is not a pristine chamber nor has a relative humidity of over 300% been observed. Therefore, something else must be contributing to the formation of water droplets. This “something else” is called cloud condensation nuclei. Cloud condensation nuclei are aerosols (small particles) in the atmosphere. Most people refer to aerosols as dust or haze when visible. These aerosols attract water vapor molecules and readily bond with them. Aerosols are very abundant and are a critical ingredient for condensation to occur.
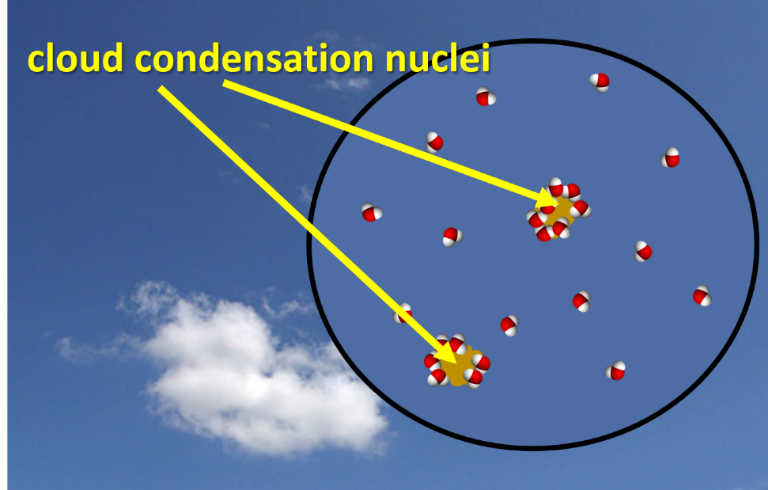
Both supersaturation and cloud condensation nuclei are required for condensation in the atmosphere. All the information in this section was for liquid water or above freezing temperatures. Condensation and deposition do occur with temperatures below zero and is the topic of the next section.
Video: Mod 4.3.2 Factors affecting condensation (4:18 min.)
This video presents the information in this section and adds some more details.
4.3.3 Factors Affecting Deposition (ice and supercooled water)
As mentioned in the previous section, aerosols act as cloud condensation nuclei in the atmosphere when temperatures are above freezing. However, supersaturation conditions can occur when conditions are below freezing. These cold conditions bring ice and vapor deposition, rather than liquid droplets and condensation, as a method for growing clouds. Colder temperatures introduce another level of complexity because water vapor does not freeze at 0oC in the atmosphere.
Supercooled water is liquid water below freezing (0oC). Ice does not spontaneously form in the atmosphere between 0oC and -4oC, but water vapor can still condense at these temperatures. Supercooled water needs ice nuclei to form ice, much like liquid water needs cloud condensation nuclei. Ice nuclei, other than ice itself, are not abundant in the atmosphere. Hence, little ice is present at -10oC and warmer. Between -10oC and -20oC, saturation conditions can lead to ice crystals or supercooled droplets. At -10oC, one ice crystal exists per one million water droplets. At -20oC, the ratio of ice to liquid water tends to be 50% or higher. All liquid has become ice when temperatures are below -40oC.
This diagram illustrates the ice and supercooled water temperature distribution:
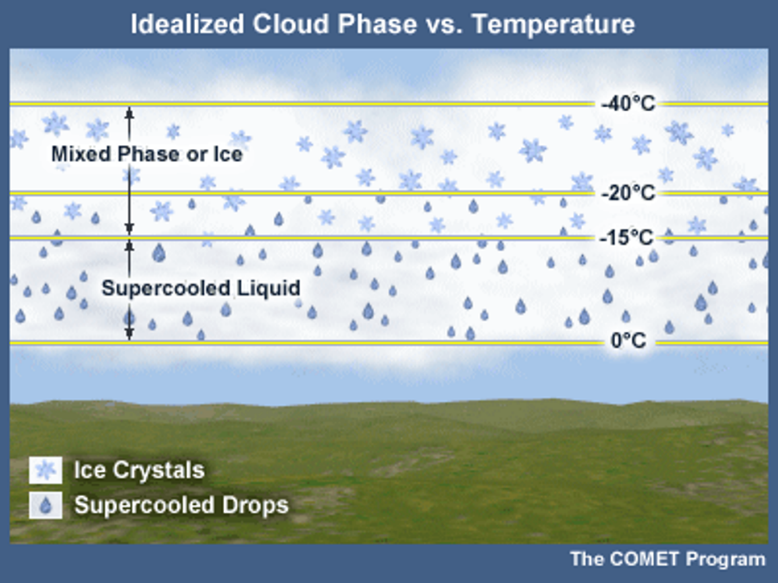
The shape of the ice crystals which form is determined by the temperature and supersaturation conditions. The six-sided molecular structure of ice causes an underlying hexagonal shape. This hexagonal configuration maximizes the attractive forces between the molecules and minimizes the repulsive forces. These small crystal variations due to temperature help make every snowflake that reaches the ground somewhat unique.
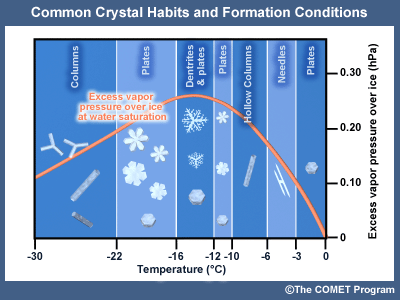
This graph shows how supersaturation (excess water vapor over ice) and temperature form various ice crystal shapes. Rapid ice formation can occur between the temperatures of -10oC to -20oC. Ice crystals can be grown under various temperature and supersaturation conditions and photographed.
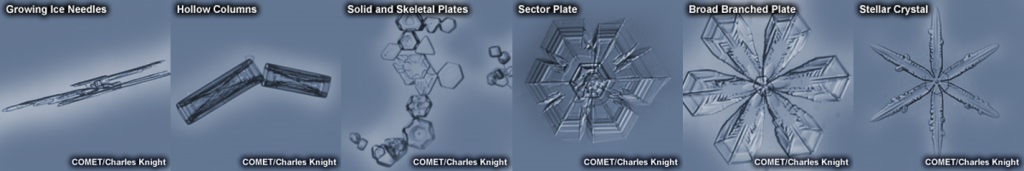
This image illustrates common ice crystal shapes.
Video: Mod 4.3.3 Factors affecting deposition (3:38 min.)
This video examines the details associated with supercooled water and ice in the atmosphere.
References:
contrail.jpg – Public domain from Wikimedia Commons at https://commons.wikimedia.org/wiki/File:Contrail.fourengined.arp.jpg
shower example.jpg – Public domain from Wikimedia Commons at https://commons.wikimedia.org/wiki/File:Il_dolce_corpo_di_Deborah_(1968)_-_Carroll_Baker_(1).jpg Clipped this image.
water_molecule.pgn used in condensation nuclei illustration – Public domain from WikiCommons at https://commons.wikimedia.org/wiki/File:Water_molecule_3D.svg
supersat and droplet size.png – From ” Nucleation of Liquid Droplets” by Roland Stull, LibreTexts is licensed under CC BY-NC-SA . Found at https://geo.libretexts.org/Bookshelves/Meteorology/Book%3A_Practical_Meteorology_(Stull)/07%3A_Precipitation_Processes/7.02%3A_Nucleation_of_Liquid_Droplets
Some text from “The COMET Program” and is found at https://www.meted.ucar.edu/norlat/snow/micro_ice/1.1.crystal_growth.htm . This material can only be accessed once logged into the COMET web site. Accounts are free to obtain.

