2.4 Earth’s Energy Balance (Shortwave and Longwave Radiation)
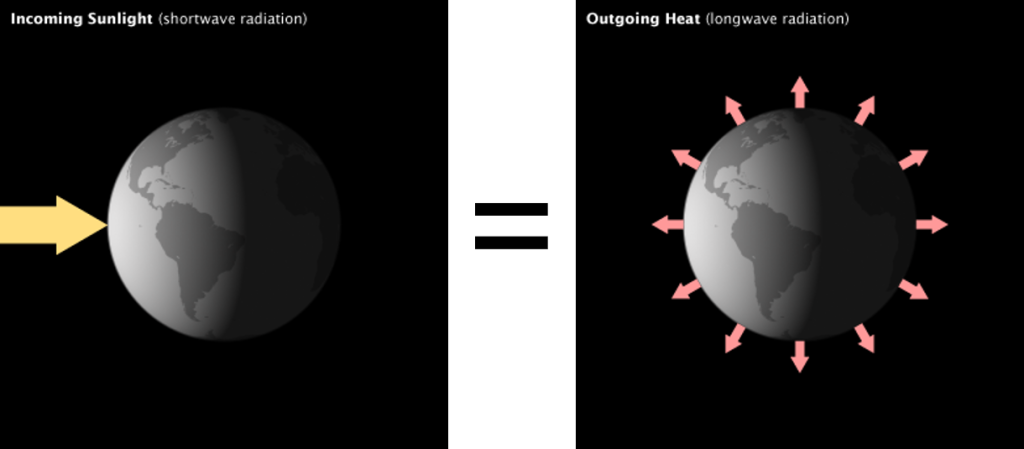
For the Earth to remain at a constant temperature the incoming radiation must equal the outgoing radiation. If incoming exceeds outgoing, the Earth will warm. If outgoing exceeds incoming, the Earth will cool. This module examines Earth’s energy balance from the perspective of keeping the Earth at constant temperature in that incoming equals outgoing.
Video: NASA: Why Does the Sun Matter for Earth’s Energy Budget?
(1:39 min.)
This NASA video introduces the concept of Earth’s energy balance.
2.4.4 Greenhouse Effect
The name “greenhouse effect” was dubbed in the early 1800s when it was thought that greenhouses stayed warmer because the panes of glass allowed solar radiation to enter, but prevented radiation emitted from plants and other objects inside the greenhouse from escaping. Actually, the reason why greenhouses stay warmer inside has to do with the fact that the air inside cannot mix with cooler air outside. The warm air in a greenhouse essentially gets trapped inside the panes of glass.
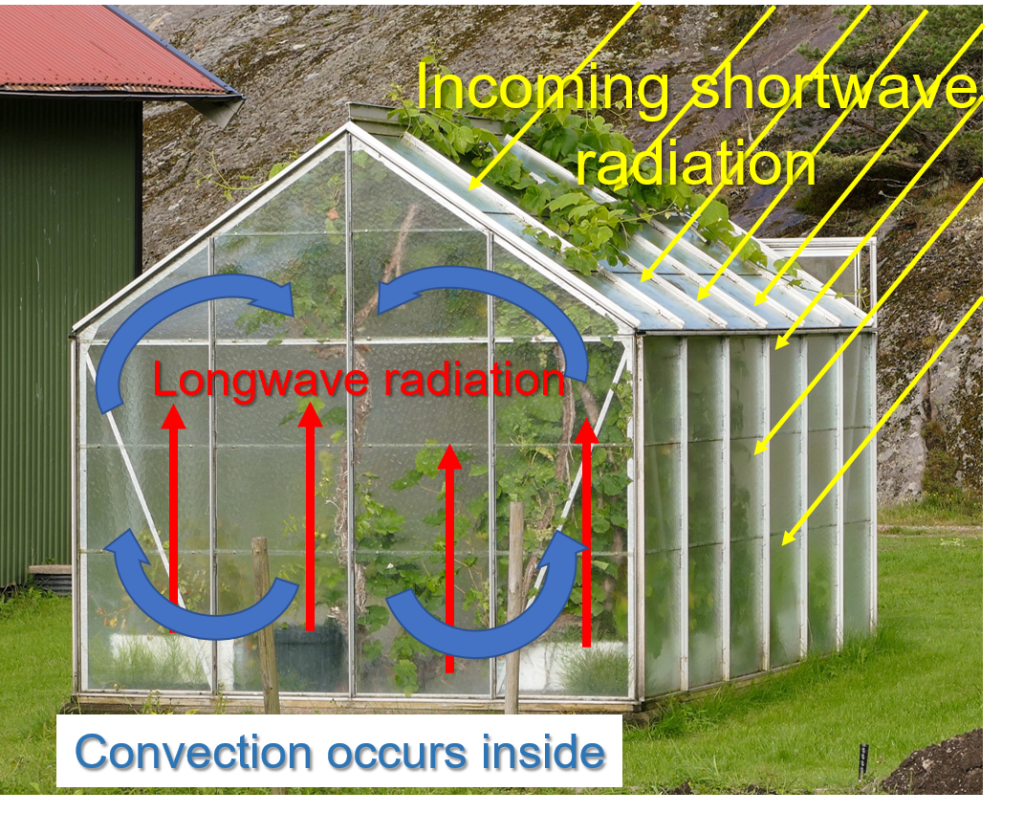
The atmospheric “greenhouse effect” is all about the absorption and emission of radiation by atmospheric gases. The physical processes in a greenhouse are much different than the atmospheric “greenhouse effect”. The variable gases associated with capturing heat are now referred to as greenhouse gases (GHGs), which indicates that the greenhouse terminology will be used for decades.
Video: 1.1.2 Earth’s Energy Budget (7:00 min.)
This video from the University of Oklahoma covers several concepts in this module, including the greenhouse effect.
References:
incoming equals outgoing.png – Public domain diagrams from NASA at https://earthobservatory.nasa.gov/features/EnergyBalance/page1.php
incoming energy details.jpg – From NOAA, NWS public domain at https://www.weather.gov/jetstream/energy Modified image in this module to only display the incoming portion.
outgoing energy details.jpg – From NOAA, NWS public domain at https://www.weather.gov/jetstream/energy Modified image in this module to only display the outgoing portion.

