1.6 Atmospheric Pressure
Atmospheric pressure is the weight in an air column that extends from an area on the ground to the top of the atmosphere. This weight is caused by gravity pulling gas molecules towards the Earth. At sea level, the weight of a column on one square inch of area is roughly 14.7 pounds, resulting in an air pressure of 14.7 pounds per square inch. Think about this value. An average human body has a surface area of about 3000 square inches. Thus, your body has a force of over 40,000 pounds of air pressure on it. Right now!
Measuring Air Pressure
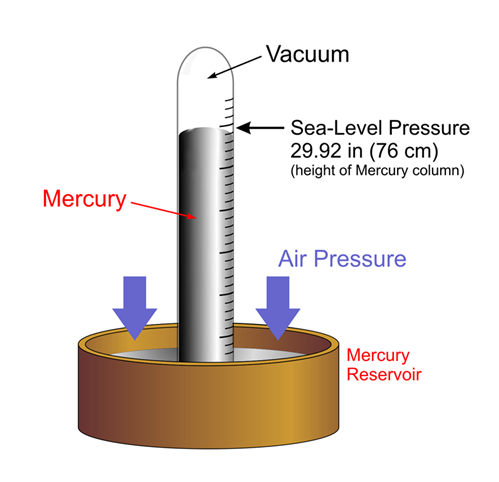
One of the first instruments to measure air pressure was the mercurial barometer. A mercurial barometer has a dish of mercury with a vertical tube placed in it. As the atmospheric pressure changes, the force exerted on the mercury changes and the level of mercury in the tube changes.
Mercurial barometers are extremely accurate and reliable, although the toxicity of the mercury has caused them to be discontinued. However, many weather observations still traditionally report air pressure in inches, which refers to the height of a mercury column, rather than an actual pressure value like millibars.
Air Density
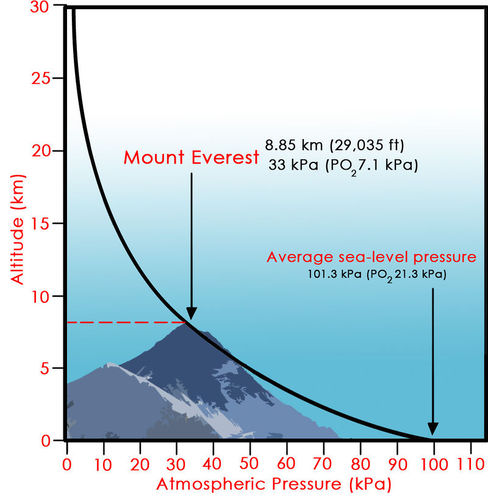
Since air is a compressible gas, the density of gas molecules in a valley is higher than at a mountaintop location. A valley location has more atmosphere over it and hence a higher atmospheric pressure. This characteristic is why physical exertion at a higher elevation will cause a person to breathe more. A body recognizes the lower density of oxygen molecules at the higher elevation. This graph illustrates this change in atmospheric pressure with height.
Pressure decreases about 50% for every 5 km (16,400 feet). Likewise, the density of air decreases as altitude increases. A person can think of air being thinner (less dense) on a mountain, which is correct. Pressure is much easier to measure than density, so pressure values are used universally in the atmospheric sciences.